[latex]\Delta G=\Delta G^{\circ }+RT\text{ln}Q[/latex] R is the gas constant (8.314 J/K mol), T is the kelvin or absolute temperature, and Q is the reaction quotient. We may use this equation to predict the spontaneity for a process under any given set of conditions as illustrated in Example 5.. Δ f G = Δ f G˚ + RT ln Q f, where Q f is the reaction quotient. At equilibrium, Δ f G = 0, and Q f = K, so the equation becomes Δ f G˚ = −RT ln K, where K is the equilibrium constant of the formation reaction of the substance from the elements in their standard states. Graphical interpretation by Gibbs. Gibbs free energy was originally.

Delta G Solved Calculate Delta G Degree R For The Reaction Co G Chegg Com downloadjokerlaugh
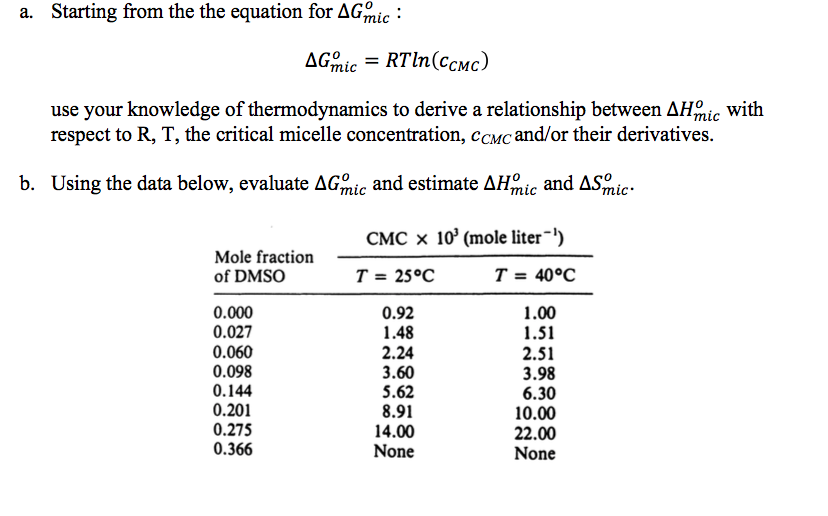
Starting from the equation for Delta G degree _mic

Calculating delta G the thermodynamics of transport YouTube
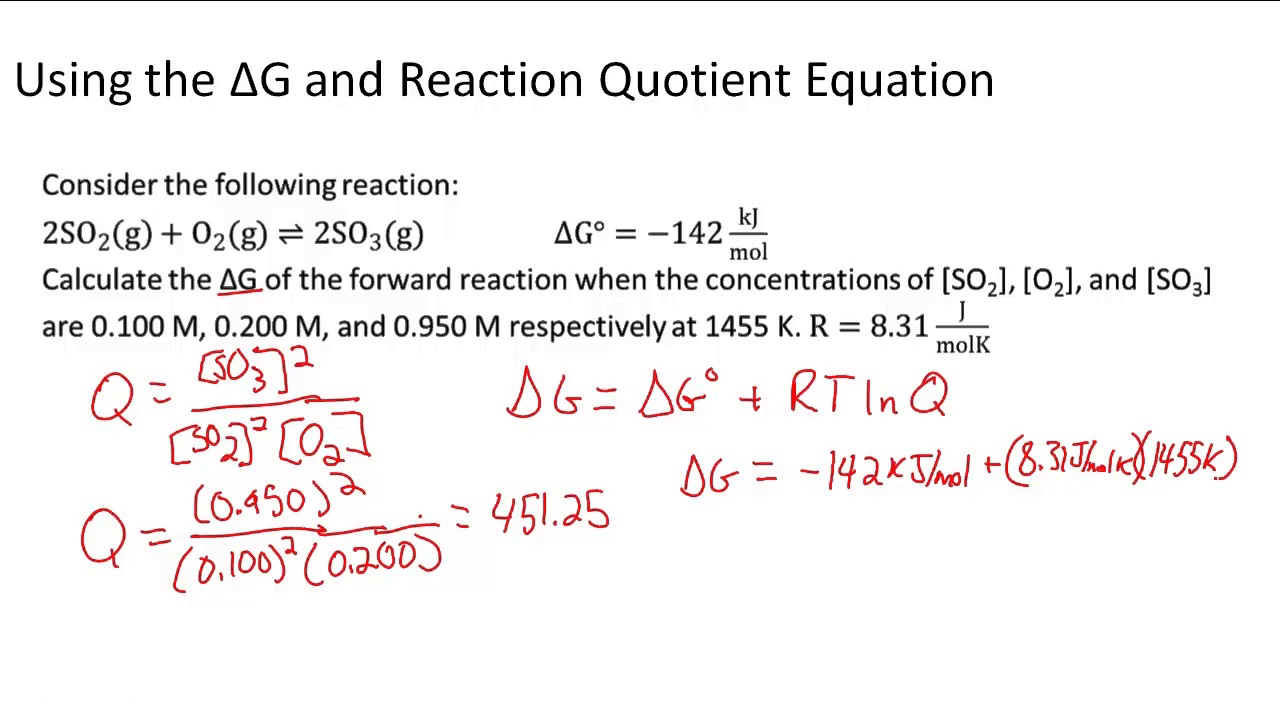
Solving problems involving ΔG = ΔG° + RT ln Q YouTube
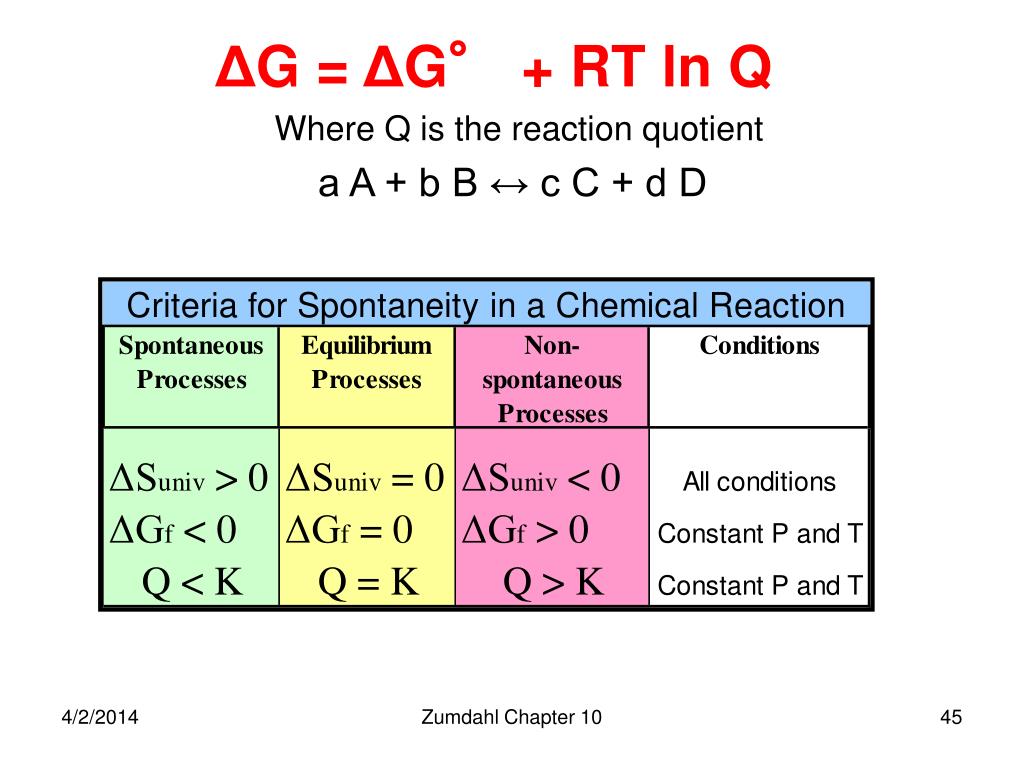
PPT Chapter 15 Spontaneity, Entropy, and Free Energy PowerPoint Presentation ID652404
![Solved H IIF ] HF] ΔG。=RT ln K =(8.3 145 JK.molX298 K) Solved H IIF ] HF] ΔG。=RT ln K =(8.3 145 JK.molX298 K)](https://d2vlcm61l7u1fs.cloudfront.net/media/5e4/5e421731-72e6-4a60-b3ee-2c9542c29ee7/phpIeCQEU.png)
Solved H IIF ] HF] ΔG。=RT ln K =(8.3 145 JK.molX298 K)
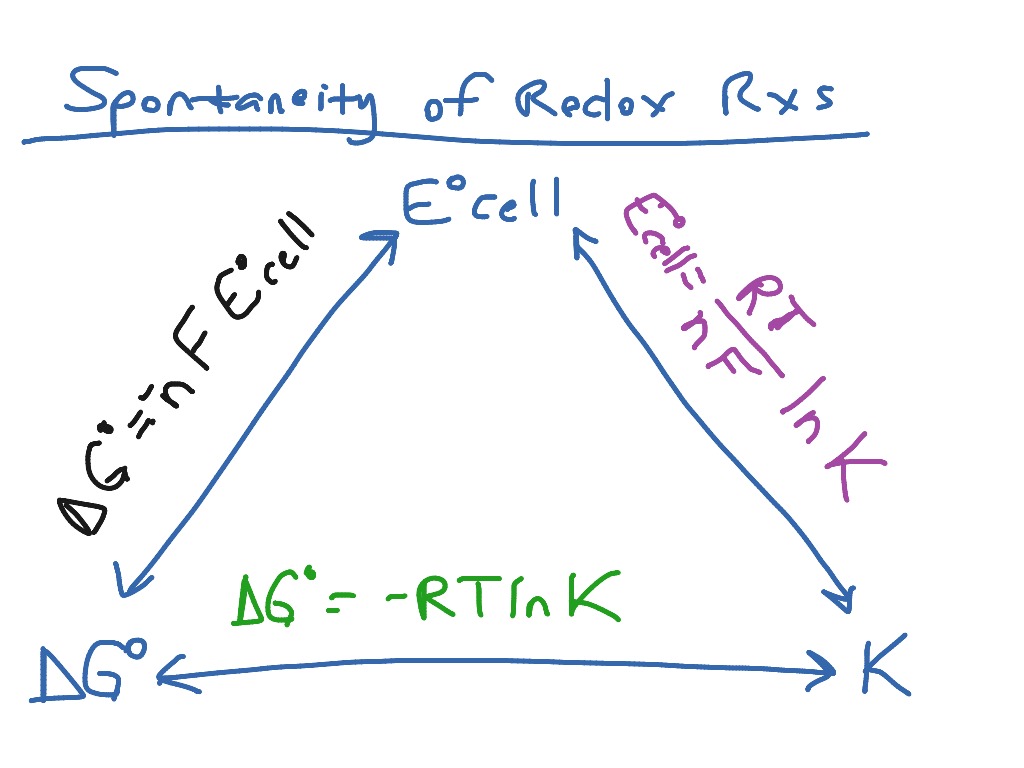
Ecell, Delta G, and the equilibrium constant Science, AP Chemistry ShowMe

Solved Given Delta G = delta G degree + RT ln Q With
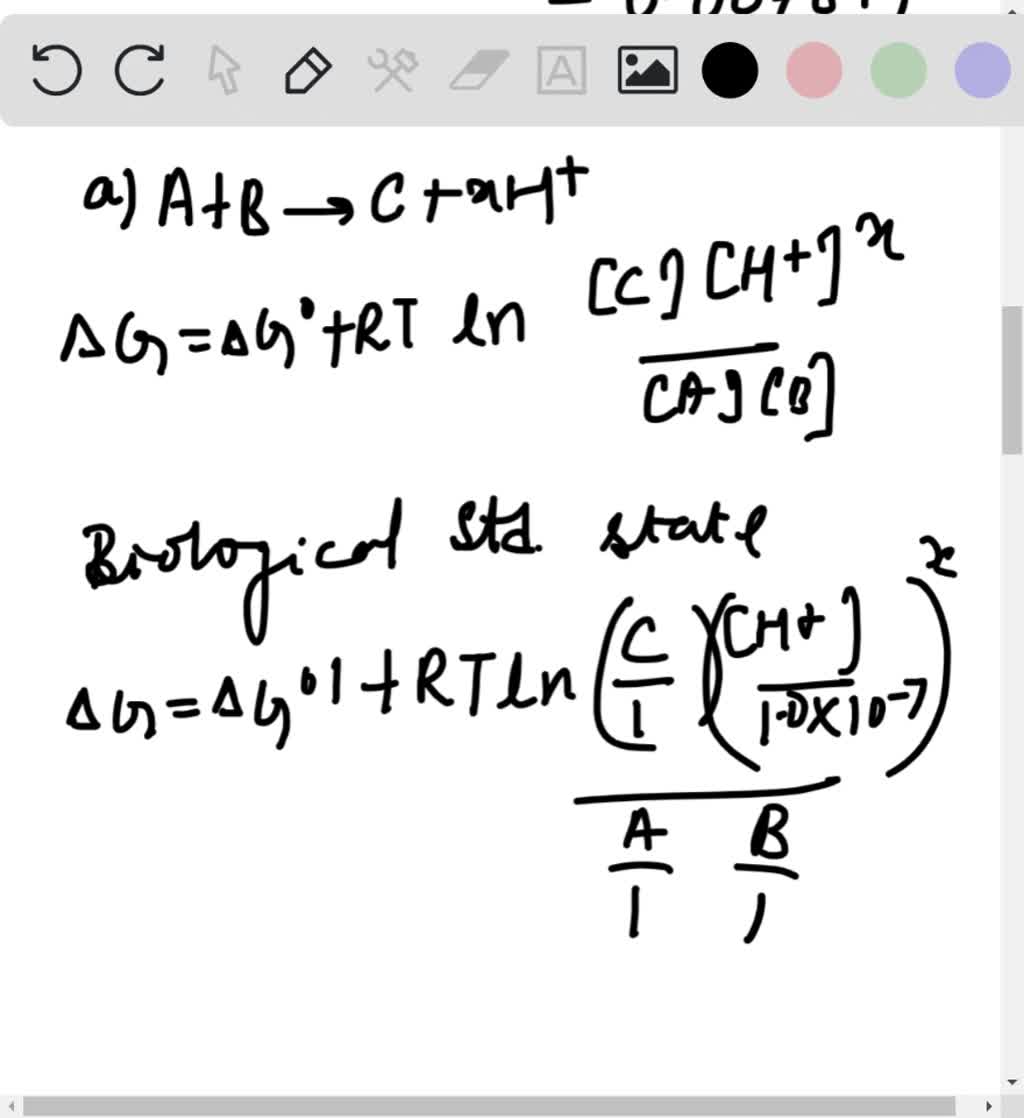
SOLVED1. the formula Delta G knot = RT ln(Keq) is for standard conditions. However, I have

Free Energy (delta G) and Equilibrium (Pt 8) YouTube

Calculating K from DeltaG (Equilibrium Constant) YouTube

SOLVEDThe equation ΔG^∘=R T lnK relates the value of Kp, not Kc, to ΔG^* for gasphase

18.3 Gibbs Free Energy and the Relationship between Delta G, Delta H, & Delta S General
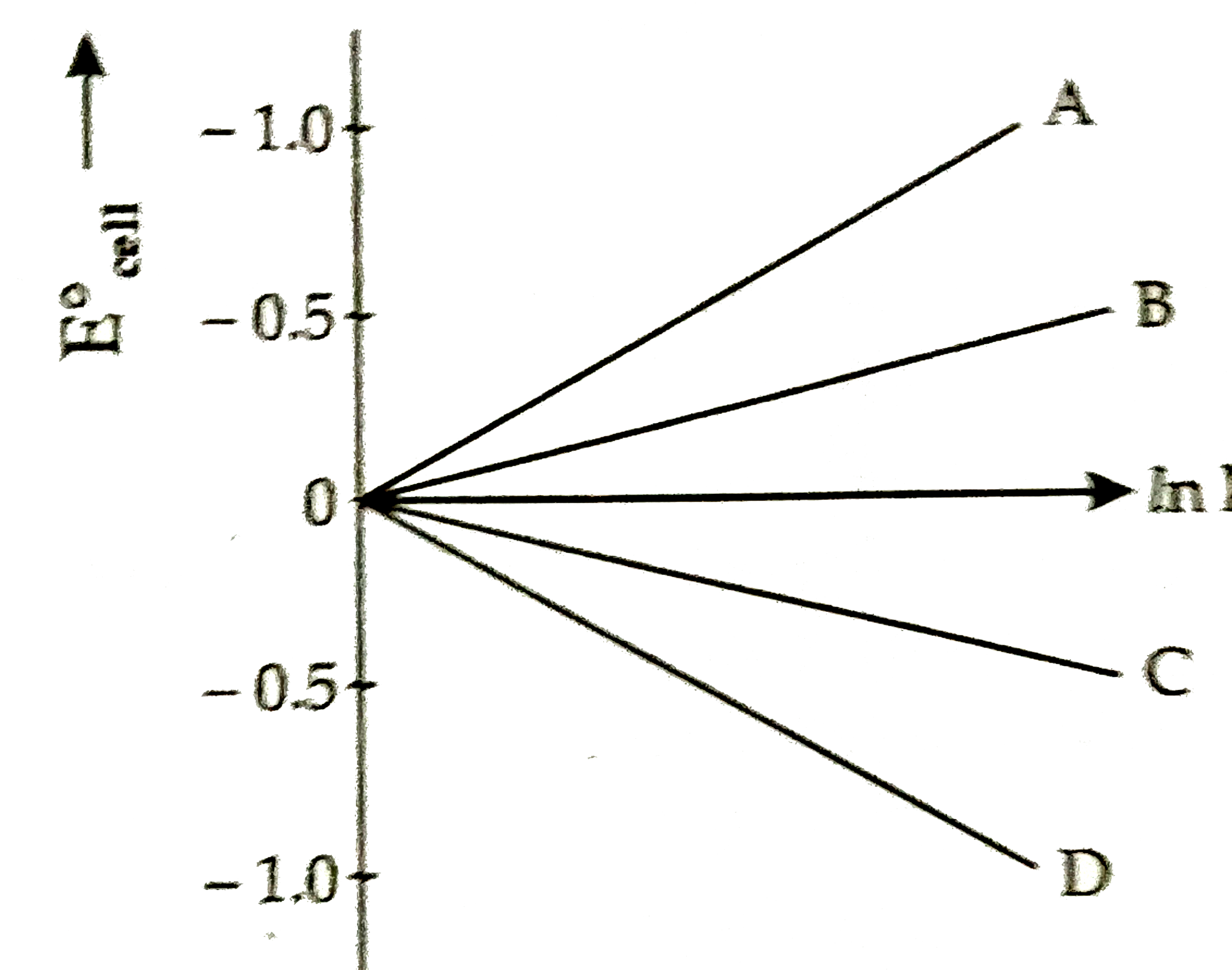
Given `Delta G^() = n F E_("cell")^()` and `DeltaG^() = RT` ln K The value of n = 2 will

D41.1 Relationships among ΔG°, K°, and E° Chemistry 109 Fall 2021
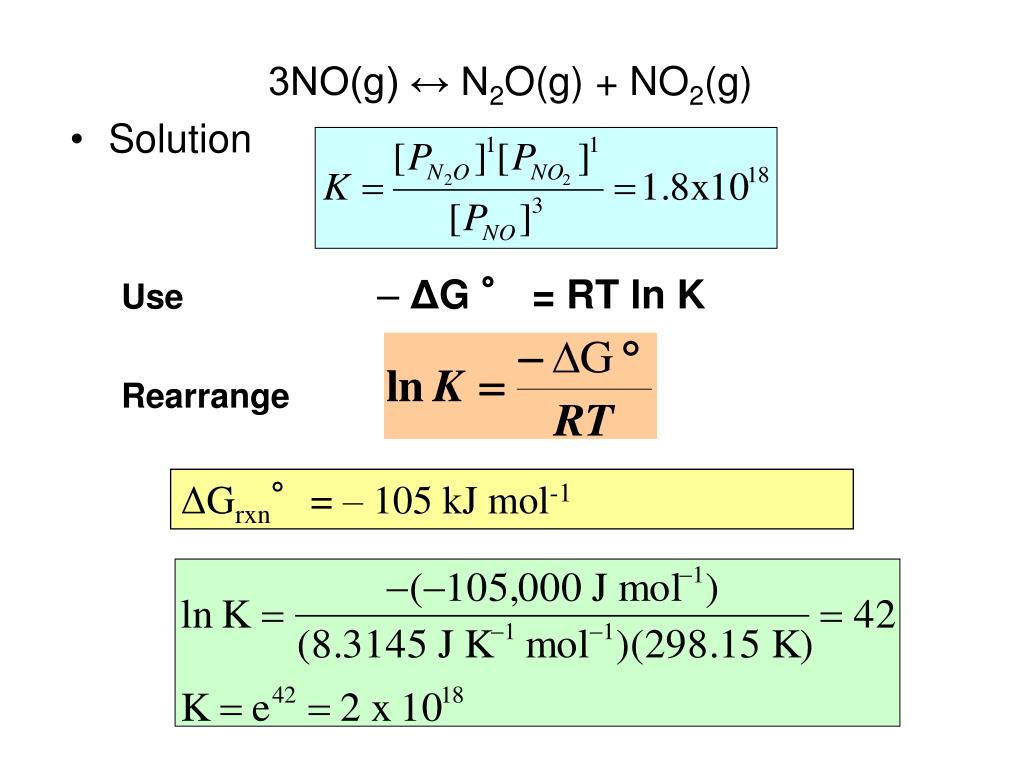
PPT Chapter 15 Spontaneity, Entropy, and Free Energy PowerPoint Presentation ID4552930

Aquí está el secreto de la vida, en esta ecuación ΔG = RT ln (C1/C2) + ZFΔV. Que quiere decir
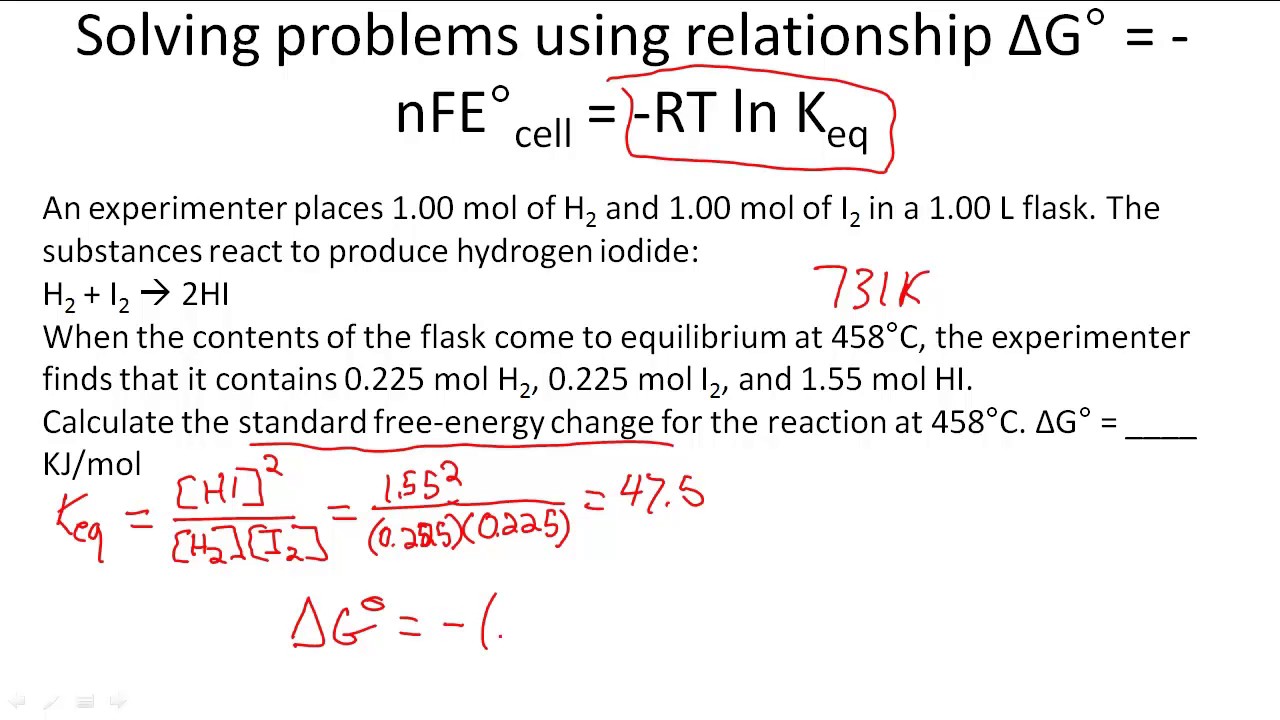
Solving problems using relationship ΔG° = nFE°cell = RT ln Keq YouTube

Gibbs Free Energy — Definition & Overview Expii
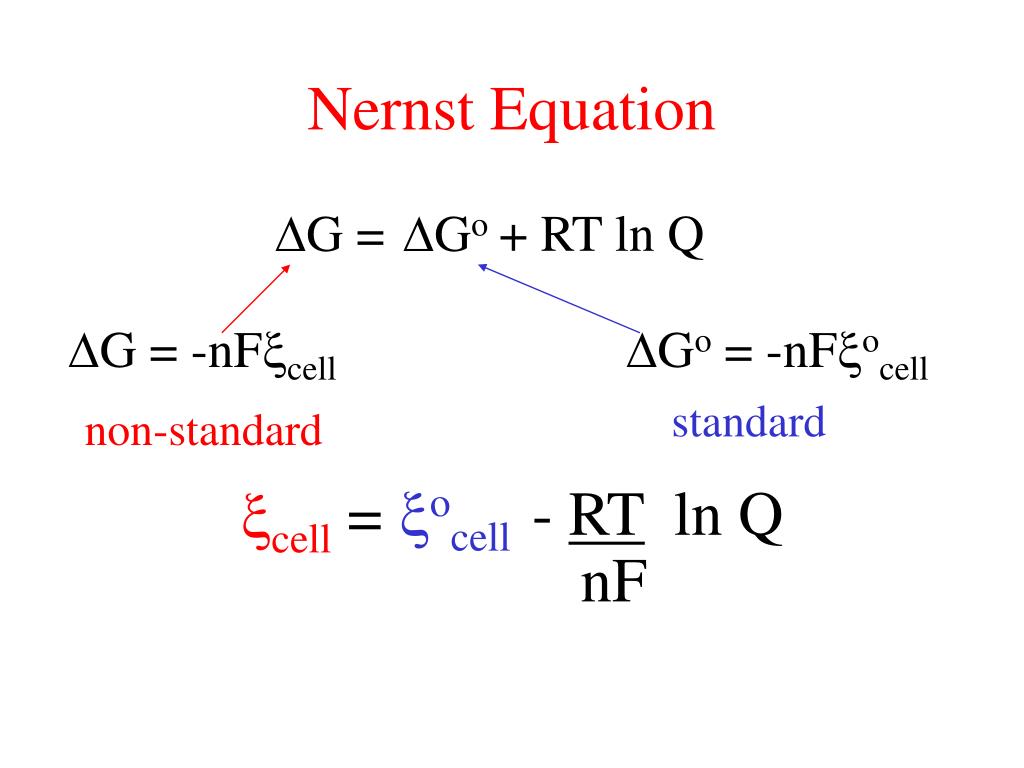
PPT Nernst Equation PowerPoint Presentation, free download ID6200903
ΔG=ΔG°+RTln(Kp) If I understand it correctly, the ΔG in this equation gives the "distance" in Gibbs free energy of a chemical system at a particular moment in a reaction (encoded by the Kp) to the gibbs free energy of the chemical system at equilibrium.. The equation that allows us to calculate Δ G is as follows: \[\mathrm{∆G= -RT\ln K + RT\ln Q\: or\: \Delta G= \Delta G^o + RT\ln Q}\] The term Q is an expression that looks exactly like the equilibrium constant expression except that it is evaluated using the starting non-standard state concentrations.